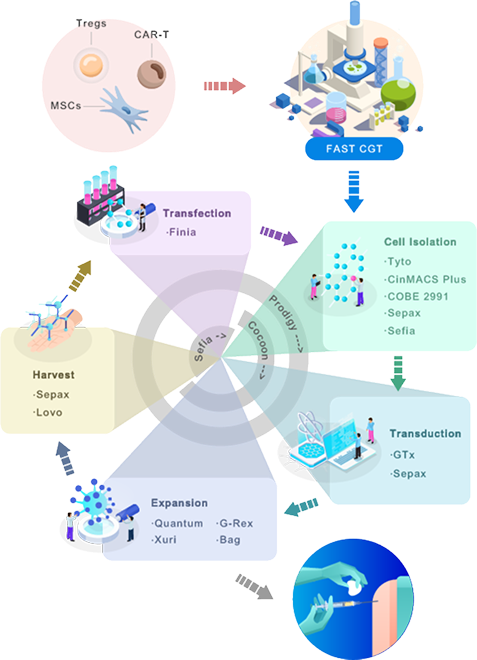
Integrating cell therapy R&D and clinical applications through the FAST CGT platform to accelerate the development and commercialization
TDM (Therapeutics Design and Manufacturing) is a novel advanced therapy development service model developed by Taiwan Bio, designed to address critical bottlenecks commonly faced in the cell therapy industry, specifically those resulting from technology transfer failures or cell therapy products inadequately designed for clinical application.
TDM integrates the mechanism of action identified by researchers in the laboratory with clinical translation requirements and utilizes the FAST CGT platform to streamline the entire “needle-to-needle” process. By incorporating clinical considerations early in therapeutic design, TDM significantly lowers the barriers for medical centers to adopt cell therapies.
Additionally, through an omics data-assisted, multi-station automated manufacturing platform, TDM seamlessly bridges research, process development, and manufacturing, accelerating the transition of cell therapy products from early research into clinical application and commercialization.
TDM integrates the mechanism of action identified by researchers in the laboratory with clinical translation requirements and utilizes the FAST CGT platform to streamline the entire “needle-to-needle” process. By incorporating clinical considerations early in therapeutic design, TDM significantly lowers the barriers for medical centers to adopt cell therapies.
Additionally, through an omics data-assisted, multi-station automated manufacturing platform, TDM seamlessly bridges research, process development, and manufacturing, accelerating the transition of cell therapy products from early research into clinical application and commercialization.